22 Which of the Following Has the Largest Ionic Radius
A one that is composed of a metal from the far left of the periodic table and a nonmetal from the far right of the periodic table. Group by having Lithium and it is Sodium and we know when you move along the period justice decreases due to increase in effective nuclear charge find know if you see it over here that you fluorine over here and nitrogen no doubt your Sodium and Aluminium has the greater size and nitrogen and Fury talking about this to talk about it the more negative charge is present on your.

Which Of The Following Is The Largest Ion Youtube
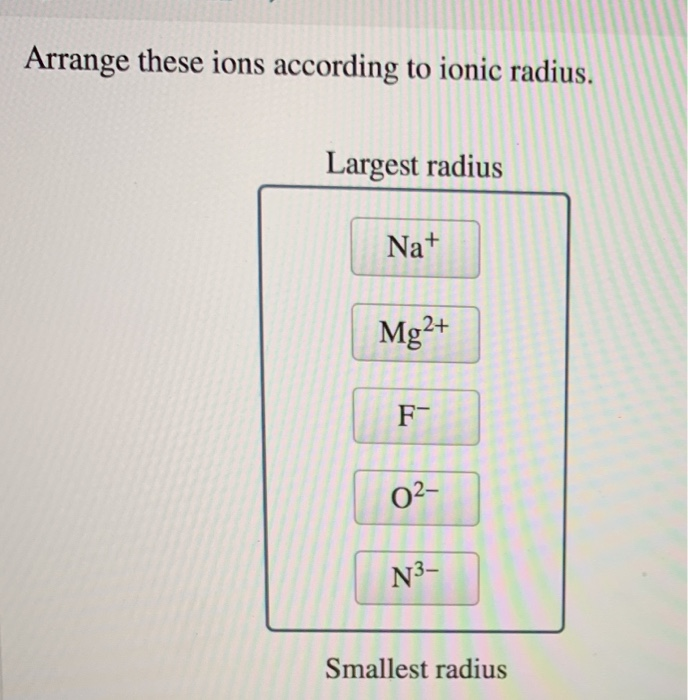
N3- has the largest ionic radius and Mg2 has the lowest ionic radius.
. The correct sequence which shows decreasing order of the ionic radii of the element is asked Apr 3 2018 in Chemistry by paayal 147k points periodic properties. Hence Cs ion will be the largest among given IA group ions ie Na Li and K. Reason The magnitude of effective nuclear charge of the outershell electrons in A l 3 is greater than that in N a.
Li F O 2 B 3 Answer. That is all three ions contain 18 electrons but have different nuclear charges. Electron clouds dont have definite edges.
Get an answer for Which of the following atoms would have the largest ionic radius. Ne E O 2-Answer. Oxygen falls left to the Fluorine and also has one extra negative charge O 2 2 than Fluorine ion F.
It is because within the period the outer electrons are in the same valence shell and the effective nuclear charge increases as the atomic number increases resulting in the increased attraction of electrons to. This is because each row adds a new electron shell. In a sodium ion there are 11 protons and 10 electrons.
Because K has the greatest nuclear charge Z 19 its radius is smallest and S2 with Z 16 has the largest radius. Belongs to the 2nd period. Terms in this set 35 Rank the following from smallest to largest.
Francium has the largest Helium has the lowest. A negatively charged ion. As reasoned above species with 18 e are larger than species with 10 e.
Na has the largest atomic radius. Since there is a larger proton to electron ratio there is more pull from the positively charged protons on the electrons pulling then closer to the nucleus and reducing atomic radius of the sodium ion. Of the following choices the largest decrease in ionic radius occurs when __________.
B S 2 Anion with 18 e. As you move down a column or group the ionic radius increases. B a solid metal.
Larger atomic number within a group larger or. In a neutral neon atom there are 10 protons and 10 electrons. The size of these species will depend upon the Z eff experienced by the valence electrons in each case note that the number of valence electrons is the same in each case above they are 2s 2 2p 6A higher Z eff means more attraction to the.
In periodic table 3rd period will have larger atomic radius than the second period. Hence the largest species is either Cl or S 2. C Na Cation with 10 e.
Which one of the following ions has the highest value of ionic radius. Mg becomes Mg Na becomes Na Ne becomes Ne F becomes F Question. Why the radius of an atom can not be measured.
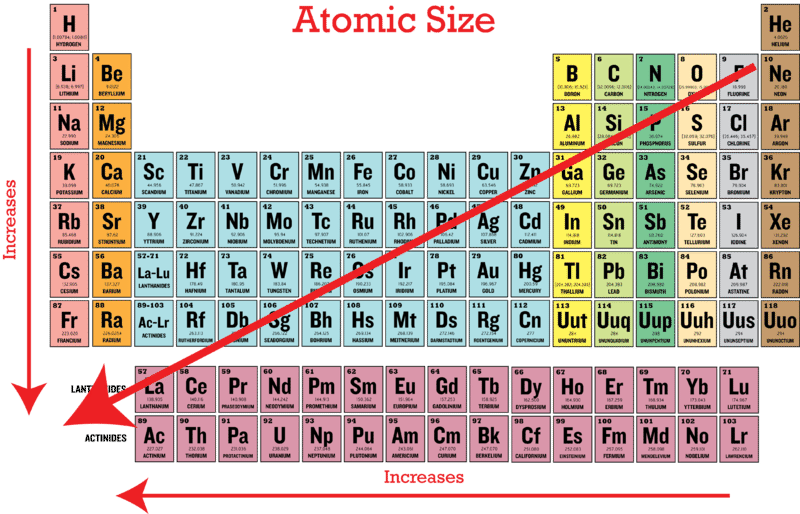
When we are thinking of isoelectronic species we are thinking of species with the same number of electrons. The size of an elements ionic radius follows a predictable trend on the periodic table. The atomic size generally decreases across a period for the elements of the second period.
2 What happens to the orbitals and thus to atoms or ions with a given number of electrons let us say 18 electrons when you increase the atomic number of the element more protons in the nucleus. S2- Cl- K or Ca2. Prior to its discovery it was referred to as eka-caesium.
View solution K C l. So they will have less ionic radius than that of the anions o same period. Ionic radius decreases moving from left to right across a row or period.
The ionic radii of elements exhibit the same trend as the atomic radii. Na and Cl. Belong to the 3rd period.
Indicate which element of the following pairs is the most electronegative. This can be explained on the basis of ze where as ze ratio increases the size decreases and when ze ratio decreases the size increases. C one that is composed of only nonmetals.
E There is no general rule to predict covalency in bonds. Which ion has the largest atomic radius. Homework 21-22 and quiz.
Of the following choices the largest decrease in ionic radius occurs when _____. Show activity on this post. Cesium and find homework help for other Science questions at eNotes.
Consider all the options-. D held together by the electrostatic forces between oppositely charged ions. Periodic Properties Which one of the following ions has the highest value of ionic radius.
K Cl and S2 form an isoelectronic series with the Ar closed-shell electron configuration. FranciumFranciumFrancium is a chemical element with the symbol Fr and atomic number 87. 1 Count the electrons all of them in each atom or ion.
Which of the following isoelectronic ions has the largest ionic radius. Which of the following has the largest ionic radius. Select the correct answer below.
Neon Ne has a bigger radius. Atomic radius increases as you go to the left and downward due to the attraction of electrons and the nucleus in an atomExplanation. Assertion N a and A l 3 are isoelectronic but the magnitude of ionic radius of A l 3 is less than that of N a.
A Cl Anion with 18 e. The order is thus Mg2 Na F- O2- N3-. Again Cl lies to the right and Na lies to the left of the periodic table.
N a B. Correct option is A Atomic and ionic radii increase from top to bottom in a group due to the inclusion of another shell at every step. In given question option A and C are cations in the second period.
For the following pairs of atoms tell which one of each pair has the largest ionic radius a. D F Anion with 10 e. Think of it this way.
Periodic Trends In Atomic Size Ck 12 Foundation
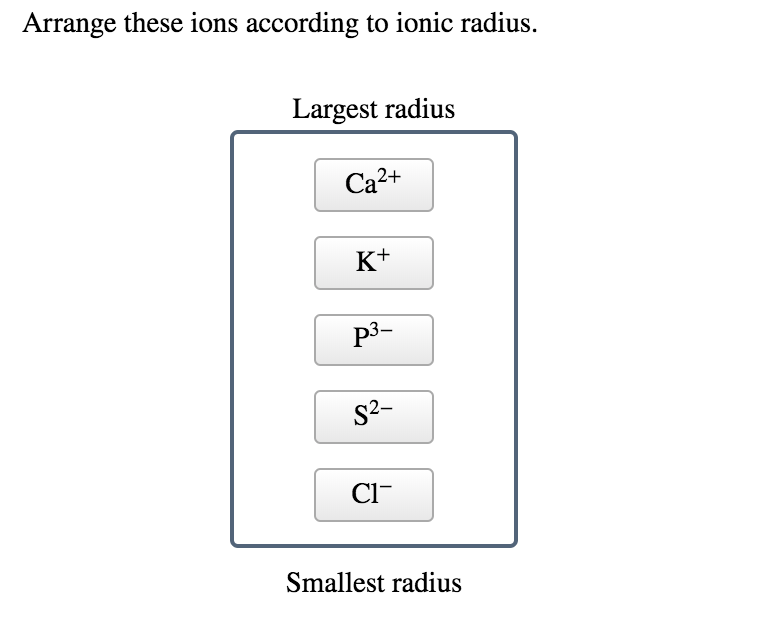
Solved Arrange These Ions According To Ionic Radius Largest Chegg Com

Solved Arrange These Ions According To Ionic Radius Largest Chegg Com
No comments for "22 Which of the Following Has the Largest Ionic Radius"
Post a Comment