Choose the Correct Lewis Structure for Br2.
Draw Lewis structure for BBr Q2. Look in the periodic table.
Br2 Lewis Structure How To Draw The Lewis Dot Structure For Dibromine Youtube
Note that Sulfur S is in Period 3 on the periodic table and can have an expanded octet and is able to have more than 8 valence electrons.

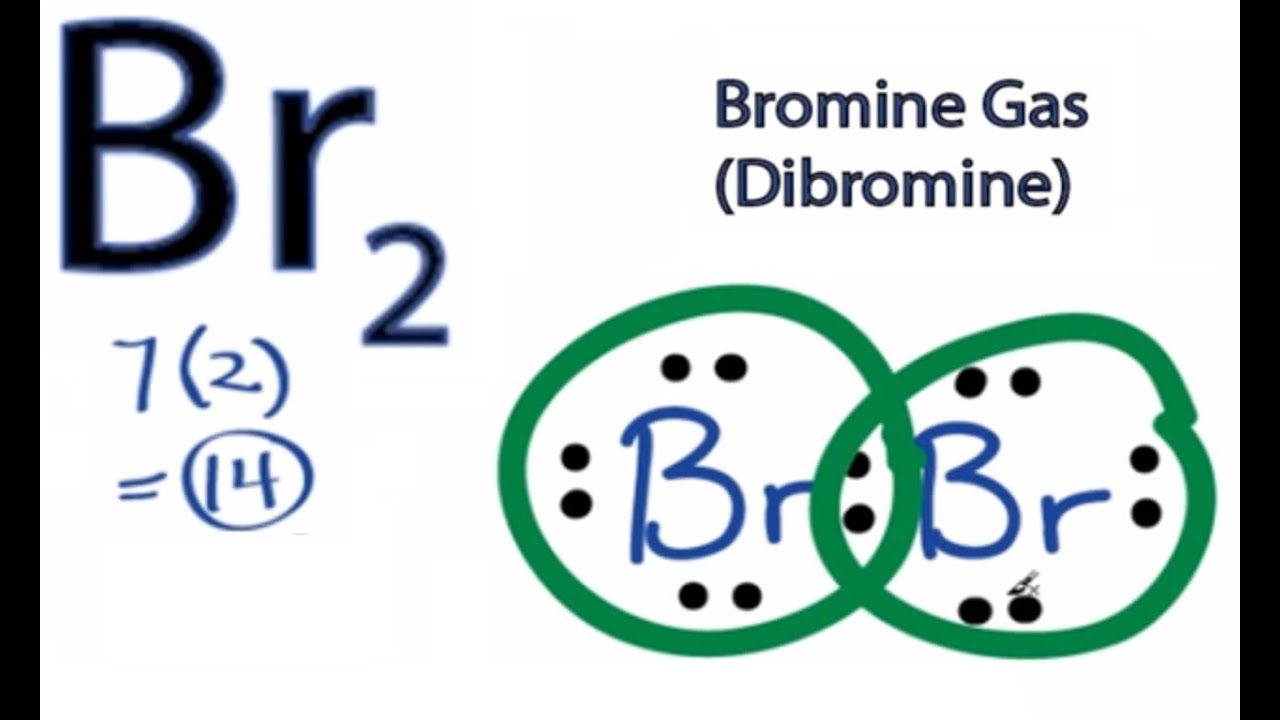
. Ten of that is the d. For the Br 2 Lewis structure there are a total of 14 valence electrons available. Lewis structure for CO.
Write the Lewis structure for a molecule of the compound. State whether BBr. What is the electron dot structure for Br2.
Choose the correct product for this reaction. The BrO-Lewis structure has a total of 14 valence electrons. First calculate the number of electrons required ER to give everybody an octet.
Lewis Dot Structure For Hbr There is a single bond connecting hydrogen and bromine. In the following Lewis structure for ClO3F chlorine has a formal charge of ____ and an oxidation number of ____. Br Br Br Br Br2 Br light Br Br C D A B.
The Lewis structure for carbon monoxide is This structures shows. There are a total of 48 valence electrons in the Lewis structure for SF6. What is the correct Lewis structure for Br2.
You need to put brackets around the BrO-Lewis structure as well as a negative charge to show that the structure is a negative ion. Start your trial now. 17 Select the correct Best Lewis structure for NOCl a reactive material used as an ionizing solvent.
The Br 2 Lewis structure is similar to F 2 Cl 2 and I 2 since F Cl and I are all in Group 7 and have 7 valence electrons. To use the Lewis Structure Calculator follow these steps. Remember that energy is absorbed when bonds are broken and released when they are formed.
Draw The Lewis Dot Structure For Hocn Cf 2cl 2 Sef 6 H 2co Study Com. Report an issue. What is the correct Lewis structure for N2.
A compound with a molar mass of about 28 gmol contains 857 carbon and 143 hydrogen by mass. State whether HBr is polar or nonpolar. CH4 g 2Cl2 g CH2Cl2 g 2HCl g Average Bond Energies C-H 413 kJmol Cl-Cl 242 kJmol H-Cl 432 kJmol C.
Drawing the Lewis Structure for PO 3 3-For the PO3 3- Lewis structure we first count the valence electrons for the PO3 3- molecule using the periodic table. Choose an expert and meet online. State whether Br2 is polar or nonpolar.
Draw the Lewis structure for AsH3. 1 An ionic bond forms between two atoms through. Draw the lewis structure for NO2- ANSWER either picture there.
A compound with a molar mass of about 42 gmol contains 857 carbon and 143 hydrogen by mass. No packages or subscriptions pay only for the time you need. The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom.
Br 2 is sometimes called diatomic Bromine since it is made up of two Bromine atoms. Which of the following is the correct Lewis structure for phosphorus tribromide PBr 3. I need some help with my chemistry homework it asks about electron dot structures.
Answer 1 of 2. What is the molecular geometr shape of the molecule for Br2 and HBr. For example if we want to obtain the Lewis structure of the Sulfate ion SO 4 2 we must first enter the charge by typing -2 or by entering -2 in the charge field and pressing the Add button.
Br 2 is a reddish gas at room temperature. A step-by-step explanation of how to draw the Br2 Lewis Dot Structure Bromine gasFor the Br2 structure use the periodic table to find the total number of. Br Br Br Br2 Br light Br Br C B B.
What is the molecular geometry shape of the molecule for BBrs Q2. Draw the Lewis structure for the H2Te molecule. Each correct drawing is worth 8 points each.
Once we know how many valence electrons there are in PO3 3- we can distribute them around the central atom and attempt to fill the outer shells of each atom. There are two Cl-Cl and two C-H bonds in CH2Cl2. A 26 B 18 C 32 D 20.
Which of the following is the correct Lewis structure for the compound PBr 3. So it doesnt matter which atom is less or more electronegative as we have to place both atoms adjacent to each other. A Lewis structure is a graphic representation of the electron distribution around atoms.
What is the lewis structure of SF6. This includes the electron represented by the negative charge in BrO-. For the SF6 Lewis structure there are a total of 12 valence electrons on the Sulfur S atom.
Which of the following is the correct Lewis structure for ethene ethylene C 2 H 4. Bromine is in column 17. What is the correct Lewis structure for Br2Br - Br.
2 lone pairs and 3 bonding pairs. ER 28 16 Then calculate how many electrons you actually have. The correct Lewis structure for a molecule of the compound C2H2 contains an alkyne bond.
Estimate the enthalpy change for the reaction below from the average bond energies given. Draw the Lewis structure for NH4 ANSWER. A Lewis structure also helps to make a prediction about the geometry of a molecule.
This is a triple bond between two C atoms. Draw Lewis structure for Br2 and HBr. Calculation of total valence electron of CO2 molecule Choose the atom with the least electronegative value atom and insert it in the center of the molecular geometry of CO2.
Enter the formula of the molecule in the field provided for it. First week only 499. There are a total of 26.
11 The total number of electrons which would be shown as dots in a correctly written Lewis structure for OF2 is. Drawing the Lewis Structure for BrO-. Which of the following elements will NOT be surrounded by an octet of electrons in a correctly drawn Lewis structure.
What is the correct Lewis structure for O2O O. Write the Lewis structure for a molecule of the compound. What is the name of the molecular geometry for this Lewis Structure.
N N. Because in these types of molecules there are only two atoms present. Provide the Lewis structures and the bolded elements oxidation number.
A step-by-step explanation of how to draw the Br Lewis Dot StructureFor the Br structure use the periodic table to find the total number of valence electron. If you want then you can avoid this step to draw the lewis structure of simple diatomic molecules like ClO- Cl2 Br2 etc. Up to 24 cash back Choose the one alternative that best completes the statement or answers the question.

How To Draw Br2 Lewis Structure Science Education And Tutorials

How To Draw Br2 Lewis Structure Science Education And Tutorials

How To Draw The Lewis Dot Structure For Br2 Diatomic Bromine Youtube
No comments for "Choose the Correct Lewis Structure for Br2."
Post a Comment