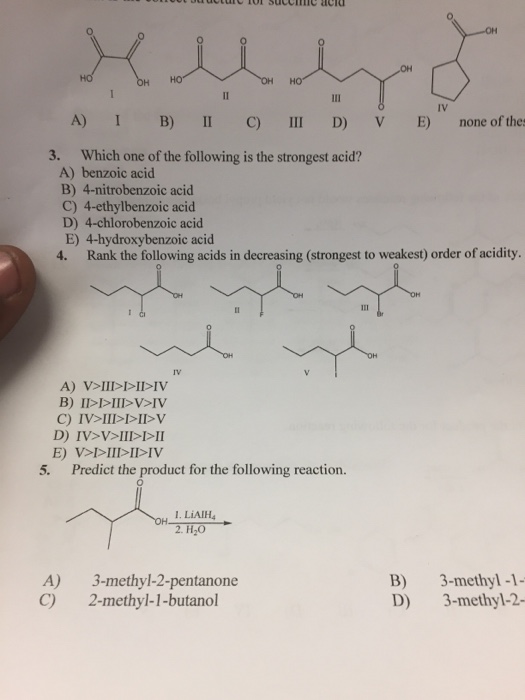
Which One of the Following Is the Strongest Acid
Since the inductive effect from the closest ortho-position is the strongest one excepts o-nitrophenol to be a stronger acid than p-nitrophenol. What is the pH after 00050 mol of HCl is added to 0450 L of this solution.

Which Of The Following Will Be The Strongest Acid
FCH2COOH is the strongest acid out of all.

. HF Ka 65 x 10-4 HFKa 65 x 10-4 Which of the following does not represent a conjugate acid-base pair. The answer to this question is. Chemistry questions and answers.
C C l 3 C O O C H C l 2 C O O C H 2 C l C O O C H 3 C O O So strongest acid is C C l 3 C O O. HCNKa 63 x 10-10 B. ACH3OCH3 bCH3CH2OH cCH3CHO dCH3CO2H.
Dont get confused between the I effect and I effect. Which one of the following is the strongest weak acid. 23-dimethylheptanoic acid 33-dichlorohexanoic acid 4-bromo-3-iodohexanoic acid 4-bromo-3-chloroheptanoic acid 4-bromo-2-fluorohexanoic acid The strongest acid is Select Select ect - is the base via Select Select to the carboxylic acid group.
A HBrO B HBrO2 C HClO2 D HClO3 E HIO. Which one of the following is the strongest weak acid. HC2H3O2OH-HC2H3O2OH-What is the pH of a solution that has H3O.
As we know the greater the number of electron withdrawing groups halogens stronger will be the acid. A HCN Ka 63 A-10. Testbank Question 028a Which one of the following is the strongest acid.
Strong acid means weak conjugate base. Thus the decreasing order is. The benzoic acid is a meta directing group and chlorine as substituents at C-2 C-3 C-4 C-5 and C-6 make the compound the strongest acid.
Which one of the following will give a solution with a pH 7 but is not an Arrhenius base in the strict sense. So according to the options given here well send B. Consider two solutions of the weak acid HCN one with concentration 010 M and one with concentration 0010 M.
A 44-dichlorobutanoic acid Ob 22-dichlorobutanoic acid OC 23-dichlorobutanoic acid d 34-dichlorobutanoic acid e 33-dichlorobutanoic acid. CCl_3COOH CHCl_2COOH CH_2ClCOOH CH_3COOH. BrCH 2 CO 2 H.
Which one of the following is the strongest acid. However the intramolecular hydrogen bond in o-nitrophenol must be broken to release proton and this requires some energy. Ka 180 105 pH 4806.
Select the strongest acid from the following list. O 4-bromo-3-iodohexanoic acid O23-dimethylheptanoic acid 4-bromo-3-chloroheptanoic acid O 4-bromo-2-fluorohexanoic acid O 33-dichlorohexanoic acid. Select the statements that correctly describe these solutions.
ICH 2 CO 2 H. So the strongest acid is CCl_3COOH. Since chlorine is an electron withdrawing group having negative inductive effect the acidity of the compound increases when attached to a more withdrawing group.
Which of the following compounds is the strongest acid. Which of the following is the strongest acid. A buffer is prepared by mixing 206 mL of 0452 M HCl and 0500 L of 0400 M sodium acetate NaC2H3O2.
Solved 10 Which One Of The Following Is A Strong Acid S 10 Key Concepts To Grasp About Ketones Aldehydes And Compare Acidic Strength Of The Following Class 12 Which Of The Following Is Strongest Acid And Why Please No Sign Of Decline In The Number Of Acid Attacks Against Which Is The Strongest Acid. Choose your ans your choice using the correct descriptors for each of the 5 drop dow the following statement true. HClO Ka 30 x 10-8 CHNO2 Ka 45 x 10-4 D.
Calculate the molarity of a solution containing 180 moles. Access a diverse Question Bank and ask You Own Doubt Now. Is the the strongest as seed here actually the strongest still here.
Thus the order of acidity is. Therefore the correct option is D. Which one of the following group-15 hydride is the strongest reducing agent.
Chloric acid denoted by the chemical formula HClO3 Perchloric acid denoted by the chemical formula HClO4 These acids are classified as strong acids because they almost completely dissociate into their constituent ions which includes their anionic conjugate base and a proton when they are dissolved in water. A ClCH2COOH B BrCH2COOH C FCH2COOH D ICH2COOH Option C Advertisement Advertisement New questions in Chemistry. H of seven B 8 correct answer option B.
Strength of Conjugate base order. ClCH 2 CO 2 H. Which one of the following is the strongest acid and why.
Which of the following is the strongest acid. The compound E is the strongest acid. A H2CO3 B H2SO3 C H2SO4 D H3PO4 E CH3COOH.
A buffer that contains 105 M base B and 0700 M of its conjugate acid BH has a pH of 9250. Chemistry questions and answers. Which one of the following is a strong acid.
Since the electron withdrawing inductive effect -I effect of the halogens decreases in the order of FCl Br I therefore the acidic strength of the α- halo acids decreases in the same order. FCH 2 CO 2 H. FCH2COOH ClCH2COOH BrCH2COOH ICH2COOH.
FCH2COOH is the strongest acid among the following.

Which Of The Following Is The Strongest Acid Youtube
Solved Which One Of The Following Is The Strongest Acid Chegg Com

Solved Which One Of The Following Is The Strongest Acid Chegg Com
No comments for "Which One of the Following Is the Strongest Acid"
Post a Comment